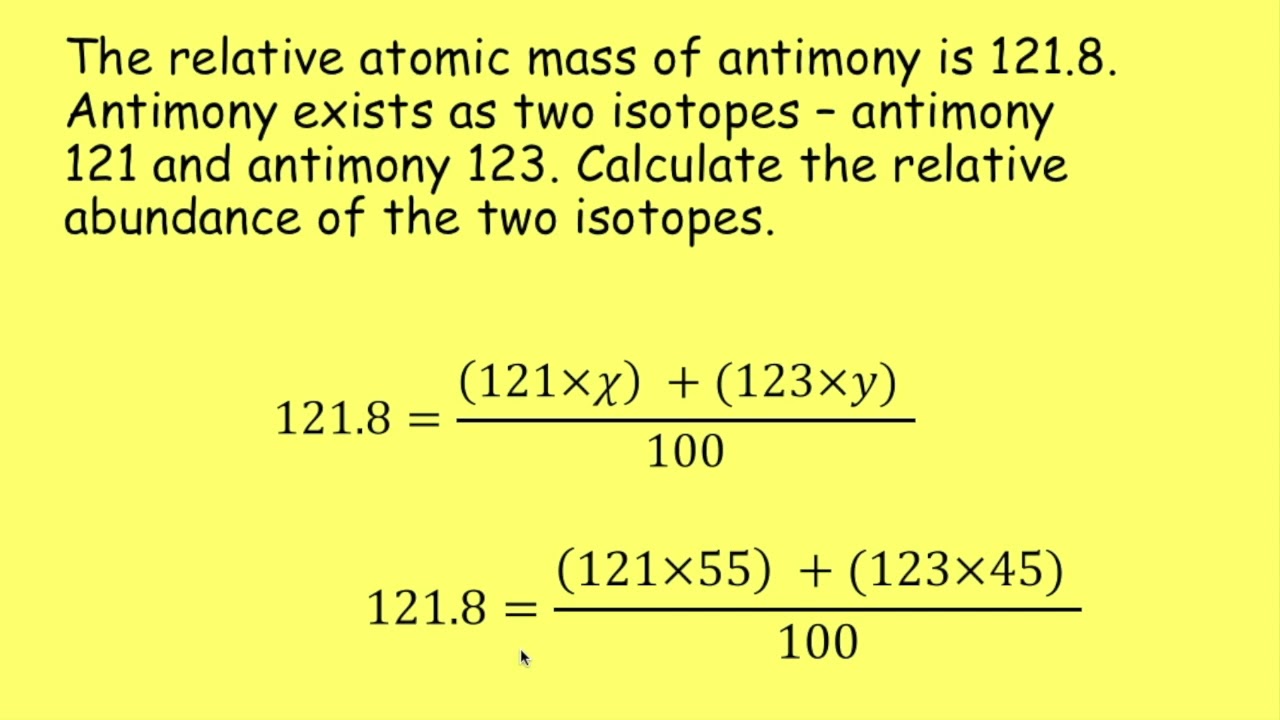
Okay, so today I’m gonna be tackling some isotopic abundance practice problems. I’ve got my notebook, a calculator, and a periodic table – let’s dive in!

Getting Started
First things first, I’m jotting down some basic formulas and concepts. You know, just to refresh my memory. It’s like, I kinda remember this stuff, but it’s always good to have a solid foundation. I wrote down the formula for calculating the average atomic mass: basically, it’s the sum of (isotope mass abundance) for each isotope. Simple enough, right?
First Problem: Chlorine
The first problem is about chlorine. It’s got two isotopes: chlorine-35 and chlorine-37. They gave me the abundances – 75.77% for chlorine-35 and 24.23% for chlorine-37. I plugged those numbers into the formula, along with their respective masses. Did the math… and boom! The average atomic mass I got is pretty close to what’s on the periodic table. That’s a good sign.
- Chlorine-35: 75.77% abundance
- Chlorine-37: 24.23% abundance
Next Up: Gallium
Next, I moved on to gallium. This one’s a bit trickier because they gave me the average atomic mass (69.72 amu) and the masses of the two isotopes (gallium-69 and gallium-71). I need to find the abundances. So, I set up an equation where ‘x’ is the abundance of gallium-69, and ‘1-x’ is the abundance of gallium-71. It is a bit like solving a puzzle. After a bit of algebra, I figured out the percentages. It took a little while to work through but I got there!
Challenge Problem: Silver
Now, for the challenge problem – silver. It is a bit of a doozy. I’ve got two isotopes, silver-107 and silver-109. They told me the average atomic mass is 107.87 amu. And, get this, they told me that silver-107 is 51.86% abundant. Seems easy at first, right? Wrong! They want me to figure out the atomic mass of silver-109, they did not give me that. I did some similar algebra like with gallium, represented the mass of silver-109 as ‘x’. I wrote out the equation using the average atomic mass formula, and after a good few minutes of scribbling and calculating, I got the answer! It was tough, but I nailed it. The mass of the isotope silver-109 is roughly 108.9 amu.
Wrapping Up
So, yeah, that’s my isotopic abundance practice session. It was actually kinda fun, like solving little puzzles. Plus, it’s satisfying to get the right answers and see how everything fits together. I think I’ve got a pretty good handle on this now. Time to put the books away and maybe watch some TV. Catch you all later!